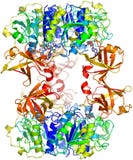
Crystal Structure Of Circadian Clock Protein Kaic At 2.8 A Resolution. Kaic Is An Essential Circadian Protein In Cyanobacteria. The Structure Resembles A Double Doughnut With A Central Pore That Is Partially Sealed At One End. The Crystal Structure Reveals Atp Binding, Inter-Subunit Organization, A Scaffold For Kai-Protein Complex Formation, The Location Of Critical Kaic Mutations, And Evolutionary Relationships To Other Proteins. A Key Auto-Phosphorylation Site On Kaic (T432) Is Identified From The Crystal Structure, And Mutation Of This Residue Abolishes Circadian Rhythmicity. The Crystal Structure Of Kaic Will Be Essential For Understanding This Circadian Clockwork And For Establishing Its Links To Global Gene Expression.
ID 6332159 © Yunxiang987 | Megapixl.com
CATEGORIES
EXCLUSIVE
Your image is downloading.
Sharing is not just caring, it's also about giving credit - add this image to your page and give credit to the talented photographer who captured it.: